 |
 |
|
|
|
In this article results of the examination of chamomile seeds microflora
are presented. Seeds of domestic and foreign cultivars of chamomile, obtained
in Institute's own production in years 1998 and 1999, were tested. Investigated
cultivars were Banatska, Novobona, Bona and Lutea. Health status of seed
samples was evaluated after incubation on moistured filter paper and, alternatively,
after incubation on potato-dextrose agar (ISTA methods), where Alternaria
spp., Penicillium spp., Aspergillus spp., Verticilium
spp., Fusarium sp. and Mucor sp. were recorded. Bearing in
mind that along with saprophytes a number of phytoparasites were found,
it could be concluded that health status of chamomile seeds must be accepted
as necessary step in the process of seed quality evaluation.
Keywords: Chamomile, seed, plant diseases, microflora.
Seed pathology as a branch of phytopathology is of recent date. Papers published so far by some outstanding scientists point out at necessity for systematic approach to actual problems. First data on health status of seed samples are based on present knowledge on seed during its germination, what is at the same time one of the most important indications of its biological value. The importance of fungi found on seed, as a factor that influence seed quality, is established very early and out of those reasons the necessity for investigation of the qualitative seed properties become prerequisite.
Chamomile as a medicinal and aromatic plant is of great importance for our country. Therefore, the investigation of its seed diseases represents a step more in effort to provide its most efficient protection.
Previous investigation in our country regarding this subject is very poor and with a brief literature review. Recently, Ministry for Protection of the Environment of the Republic of Serbia and group of scientists from the Institute for Medicinal Plant Research "Dr. Josif Pančić" have published (1999) the book entitled "Strategy of Medicinal Plants' Protection in Serbia", where the authors have also pointed out the importance of the investigation of seed health status as one of the main aims of medicinal plants' protection.
Consequently, this paper, based on investigation of the health status of chamomile seed should represent one step more in field of medicinal plants' protection.
Many fungal diseases are maintained and transferred by the seed from one to another vegetation. Sowing of infested seeds makes soils richer in parasite potential of some fungal diseases. Phytopathological analysis of seed has for its aim determination of isolated microbial species and estimation of the degree of its contamination. Conclusions based on these analyses give us a real picture on usability of the examined seed samples.
Seed samples that were the subject of this investigation, taken from the field of the Institute for Medicinal Plant Research "Dr. Josif Pančić" (what is a common practice of the Institute) were of the following cultivars: domestic Banatska and tree Slovakian cultivars Novobona, Bona, and Lutea. All cultivar were of the yields of 1998 and 1999. Analysis of the health status of chamomile seed is done with the use of standard phytopathological methods as follows:
Incubation of seed at filter paper
Incubation of seed at growing medium (PDA) and Carnation Leaf-Piece Agar (CLA)
All seed samples were analysed according to presented methods. Four hundred seeds (4 trials, each with 100 seeds) from each seed portion were sterilised (1% NaOCl) for 3 minutes and then rinsed with sterile water and transferred to Petri dishes, 15 cm in diameter. Fifty seeds from each seed portion were transferred to PDA medium following the seed surface sterilisation. After the eight-day incubation, at 25°C, parts of mycelia taken from well-developed colonies were transferred to the lateral agar in order to be further examined.
Morphological examination of the isolated fungi was conducted at monosporial
cultures. Following phenomena were monitored: speed of the growth at PDA
at 25°C, nature of aerial mycelia, presence
of pigmentation, appearance of conidiophores and conidia, manner of conidia
formation, production of chlamidospores, sometimes sclerocia, and formation
of stroma.
In every isolate 100 conidia were measured.
Identification of the present fungi was done on the basis of morphological
and growing properties of the examined isolates, and classical as well
as recent literature was used. The obtained results were processed with
the use of analysis of variance (Snedecor and Cochram, 1967).
Due to voluminous examination the determination of the fungi in most cases was done up to the level of genus. Determination up to the species level is to be a subject for some further research. At chamomile seed fungi presented in Table 1 were identified.
If the average values presented in Table1 are analysed, it can be concluded that, the most present fungi (expressed in percentages) derived from following geni: Alternaria and Fusarium. However, it should be stressed that there were no distinctive differences between the yield of 1998 and 1999, as well as between examined cultivars of chamomile.
|
|
|
|
|
| Banatska | Alternaria spp.
Aspergillus spp. Penicillium spp Fusarium moniliforme |
- 2% 2% |
2% 1% 2% |
| Novabona | Alternaria spp.
Aspergillus spp. Penicillium spp Fusarium moniliforme |
2% - 2% |
- - 2% |
| Bona | Alternaria spp.
Aspergillus spp. Penicillium spp Fusarium moniliforme |
2% - 1% |
2% - 2% |
| Lutea | Alternaria spp.
Aspergillus spp. Penicillium spp Fusarium moniliforme |
2% 3% 3% |
1% 2% 1% |
Fusarium momoliforme Sheldon
In all cultivars the presence of fungi Fusarium moniliforme was confirmed. In case the contamination was at superficial level of the seed then in common condition the seed contamination does not influence the seedling. Contaminated seeds germinate but as such they slow the growth of seedlings (Figure 1, 2).
 |
 |
|
|
|
By measuring it was determined that the seedlings germinating from contaminated seeds were for 0.7 cm shorter in comparison to those germinating from healthy seeds. In presented case the development of mycelia of the fungus Fusarium moniliforme in endosperm of germinating seed disturbs the growth of seedling due to some kind of toxic materials this fungus produce (Bilaj, 1951). If the moisture of the seed was more than 14% the more intense development of this fungus occurred.
At seeds this fungal species form abundant white-cream, powdery mycelia. Due to its presence filter paper used to be stained in indigo blue. Sporulation at seed is massive in few days, and there are microconidia.
At growing medium (PDA), at temperature of 25°C, the aerial mycelia were foccose, powdery, of dirty-white colour. Pigmentation of the agar itself was variable, no pigmentation or greyish orange in some isolates to violet grey, dark violet or dark magenta in others (Figure 3). Isolates we have obtained got light orange pigmentation. Colony growths very fast (4 cm in 3 days at 25°C).
 |
 |
|
|
|
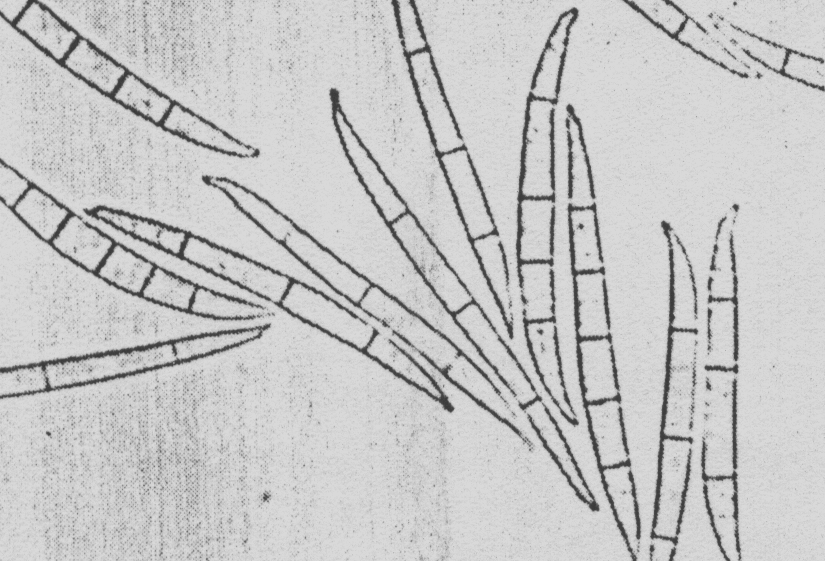
This fungus forms microconidia and macroconidia. Microconidia are formed abundantly in chains from monophialides on branched conidiophores (Figure 4).
 |
 |
|
|
|
Macroconidia are inequilaterally fusoid, delicate, thin walled, with
an elongated, often sharply curved apical cell and pedicellate basal cell.
They are 3-7 septate and measure: 25-6-40 x 2.5x4 m
(Figure 5).
Microconidia measure 5-11.5 x 1.5-2.5m and
are fusiform to clavate with a slightly flattened base, they occasionally
become 1 septate (Figure 6).
Chlamydospores are absent.
Alternaria spp.
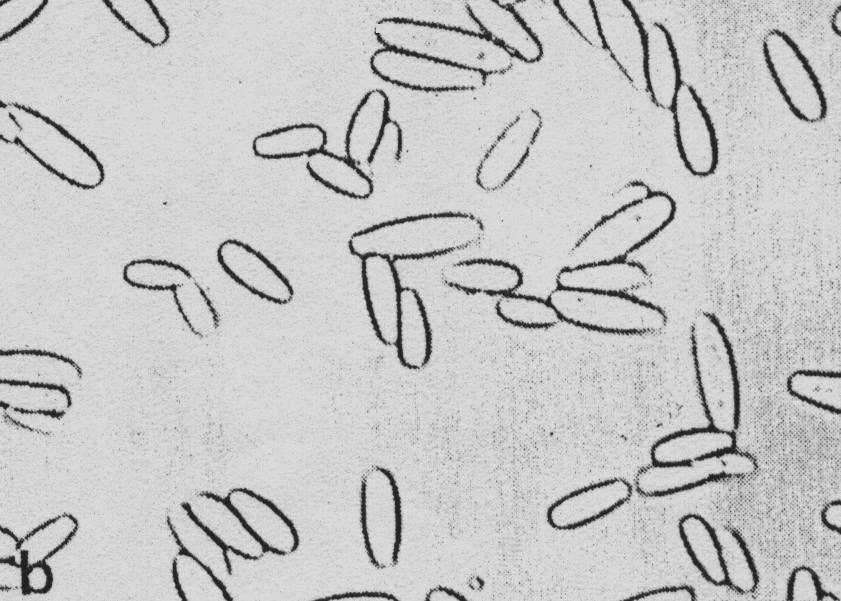
When contaminated chamomile seeds are placed on filter paper this fungus forms abundant mycelia, which is at first of dirty-white colour and later on, it becomes from dark brown to black. At contaminated seedlings abundant black covering made of conidiophores and conidia of this fungus is formed (Figure 7). Conidia are pear or spindle shaped, divided with 3-6 transversal and few longitudinal partitions. Conidia form strings (Figure 8) or they appear individually. In contaminated plant debris and on seed it maintains its vitality for the long period of time.
 |
 |
|
|
|
In most samples this fungus was found in some more occurrence at seed of the yield of 1999 in comparison to the yield of 1998. These differences may be attributed to the favourable climatic conditions for this fungus present in period from the end of April and in entire May 1999.
Chamomile seed is exposed to attack of a small number of parasites that do not have a significant influence on chamomile seed quality.
In this investigation on chamomile seed fungi deriving from following geni were identified: Alternaria, Fusarium, Penicillium and Aspergillus. For chamomile seed only Fusarium moniliforme is of importance.
If Fusarium moniliforme is present in superficial layer of seeds does not have an influence on seed germination. If present in endosperm it brings about reduction in seedlings' growth in comparison to non contaminated seed endosperm.
On chamomile seed the most often found fungal species derive from the genus Alternaria. However, there are also fungal species that derive from genus Penicillium and Aspergillus.
On the basis of the obtained results following can be concluded:
There are a small number of pathogenic fungi found on chamomile seed.
There were isolated 4 fungal species.
The most often isolated fungal species were those that derive from genus Alternaria and Fusarium.
For chamomile seed the most important fungus is Fusarium moniliforme due to its mycotoxin that has an influence on seedlings' resistance.
Bilai V.I. (1955): Fusarium, 283-284.Kiev, Ukr.SSR Acid.Sci.
Boot (1971): The genus Fusarium, 123-127. Commonwealth Mycological Institute, Kew, Surrey, England.
Gerlach & Nirenberg (1982): The genus Fusarium a pictorial atlas, 301-313. Mitteilungen ans der Biologischen Bundesanstalt fur Land-und Forstwirtschaft. Berlin-Dahlem .
Dobrozrakova T.L., Letova M.F., Stepanov K.M., Hohrjakov M.K. (1956): Opredelitel nabolestite porasteliayta, Moscow-Leningrad, Nauka, p. 246.
Kojić M.R. ed. (1997): Grupa autora: Kamilica (Chamomilla recutita (L) Rausch.), Monografska studija.
Kišgeci J., Adamović D. (1994): Gajenje lekovitog bilja, Nolit, Beograd.
Joffe A.Z. (1974): A modern system of Fusarium taxonomy.
Lester W. Burgess (1994): Laboratory manual for Fusarium research, Sydney, 60.
Malone J.P., Muskett A.E. (1964): Seed borne fungi. Plant Pathology Division, Ministry of Agriculture, The Queen's University, Belfast, p. 33.