Figure 1.
Because of degradation and overgrowing of natural ecosystems, plant biodiversity, which is essential for breeding work directed to amelioration of agricultural as well as market value characteristics, is highly endangered. The paper focuses on the main directives and significance of national research program for collecting, maintenance, preservation and evaluation of autochthonous and/or introduced genetic material of medicinal and aromatic plants in Slovenia. The first stage of the program foresees monitoring of natural populations, their characterization and "in situ" conservation of evaluated autochthonous plant material. In order to manage extensive data network in most efficient way, our working group has recently developed a relational database, named MEDPLANT, established for floristic, faunistic, taxonomic and analytic data input for medicinal and aromatic plants (MAP) of Middle European and Mediterranean regions. MEDPLANT is aimed at screening those high potential native populations of medicinal and aromatic plants, which meet raw material quality requirements in different branches of processing industries (i.e. phytoterapy, food industry, phytocosmetics). MEDPLANT system works on relational database principles: collected data are arranged according to their characters in appropriate databases (systematic, geography, habitats, pedology, phytocoenoses, chemical analyses, varieties / cultivars). Pointers, which connect specific data in these databases, enable establishing mutual relationships between required data in the network.
Slovenia is a small Central European country but despite of its small area (20.256 km2, 2 mil inhabitants), it is very rich in plants' diversity. The territory of Slovenia covers 3 centers of diversity (Mediterranean, European-Siberian, Near Eastern). However, vegetation and flora in Slovenia is considered as degraded, what can be attributed to antropogenic factors and habitat loss. That's why already in 80-ies Slovenian botanists have made efforts in making an inventory of Slovenian flora, aimed at securing basis for conservation of remaining natural resources of rare and/or endangered species. The result of this intensive research work is "The Red Data List of Threatened Vascular Plants in Slovenia" (1989), edited by Institute for the conservation of natural and cultural heritage of Slovenia. This document is based on IUCN categories and is the result of classification of threatened plants according to the degree of threat (Wraber, 1989). The Red Data List of Threatened Vascular Plants in Slovenia covers 28 plant species, (4 of which have medicinal properties - Cypripedium calceolus L., Fritillaria meleagris L., Gentiana lutea L. and Taxus baccata L.) protected by law from wild gathering.
Of approximately 3000 plant species known to be autochthonous or well adapted to Slovenian climate centuries ago, about 10% are estimated to be endangered (34 have been injured, 77 vulnerable, 192 are rare) (Aljančič et al., 1995). Among species (there are over 100 autochthonous species with potential medicinal properties), which are regarded as endangered, appear more and more frequently medicinal and aromatic plants (MAP).
International Regulations/conventions like Convention on Biological Diversity (Agenda 21 - Rio de Janeiro 1992 and Pan-European Biological and Landscape Diversity Strategy - Sofia 1995); Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES); Convention on the Conservation of European Wildlife and Natural Habitats - Bern Convention (1 June 1982), Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora (lists 10 species of MAP); Council Regulation (EC) No. 338/97 of 9 December 1996 on the protection of wild fauna and flora by regulating trade therein, obligated Slovenia to prepare national biological and landscape diversity conservation strategy and sectional implementation programs by the year 2000 and appropriate legislative background. International conventions, that were ratified in Slovenia in last 4 years (in 1996 Rio Convention and in 1999 CITES) and also national legislation (Nature conservation law, adopted in 1999) are considered as general documents, that list more than 35.000 plant or animal species, that are considered as endangered. Only about 50 plant species, covered by this legislation, belong to a group of medicinal and aromatic plants (MAP).
MAP Market characteristics in Slovenia
Being aware of the MAP market fluctuations and of the trends in the European Market, in Slovenia MAP market analysis was made in 1994. The results showed, that in Slovenia MAP were cultivated on only 20 ha mainly on a fragmented areas with minor possibility of usage of modern machinery (Baričevič et al., 1996). Consequently, cost price of domestic plants was too high to compete with imports. Total supply of herbs (drug plants) on target markets in Slovenia was estimated at 1372 t. 84.3% of this amount (1157 t) was imported (3.571.000 USD), main suppliers being Albania, Bulgaria, Czech Republic, Hungary, Poland, Russia, China, Croatia and Macedonia (manpower low price). At least 707 tons (2.406.000 USD) of these drug plants could be produced in Slovenia: thyme (Thymi herba), St. John's wort (Hyperici herba), Chamomille (Matricariae flos), sage (Salviae folium), peppermint (Menthae piperitae folium), gentian (Gentianae radix), mallow (Althaeae radix/folium), dill (Anethi fructus), coriander (Coriandri fructus), caraway (Carvi fructus). The main consumer of drug plants was condiments' processing industry (400 t), followed by phyto-pharmaceutical industry (170 t), beverage production industry (80 t), tea production industry (3 t) and cosmetic industry (0.2 t). Domestic overall demand on drug plants by processing industries was estimated at 650 t and Slovene export of drug plants at 600 t. The overall supply of drug plants (165 t wild collected, 50 t cultivated, 1157 t imported) exceeded their overall demand (1250 t). The situation was very similar or even worse when assessing MAP-based half-products (like fluid extracts, dry/dense extracts, essential oils), where import (62 t) exceeded the overall demand (domestic processing industries' demand 44 t, re-export 7.4 t). Discrepancy between the demand and supply of drug plants and MAP-based products on Slovene market showed the future need of quality-based regulation of imports, the need of enlargement of the area under MAP cultivation and of development of MAP processing facilities.
In spite of neglected acreage under MAP cultivation and relative low demand on raw materials in target markets in Slovenia, MAP were identified as minor crops that could be of national interest within sight of their cultivation as well as of marketing of their alternative products. This was the reason, why directives and the proposal of the national program for production, processing and quality control for medicinal and aromatic plants have been prepared (Baričevič et al., 1994; Baričevič and Rode, 1996). Although the program didn't receive the financial support on the whole, some parts - like National collection / Genebank for MAP has been officially recognized in 1995 and is annually financed by Ministry for agriculture, food and forestry. The program foresees the strategy for preservation of natural resources as one of the most important tasks.
Establishment of genebank for MAP
Monitoring of natural habitats of MAP in and their active maintenance/in situ, ex situ
Identification, development, approval of MAP cultivars
Assurance of quality seed supply for cultivation purposes
Establishment of reference plantations, introduction of Good Agricultural Practice
Cultivation planning (based on MAP demand quantities) and agrosystems development
Market research - local market demands according to the market segmentation (food, cosmetic, phyto-pharmaceutical industry,...)
Ecological suitability of cultivation (based on natural districts, GAP)
Propagation of plant material (Uniformity of plant material - DUS criteria
Aplication of sustainable technologies (Sustainable production)
Harvest planning (required machinery, drying facilities,...)
Quality control (QA of MAP and their half-products, WHO guidelines 1998)
Cultivation economy calculation
Assurance of drying and processing facilities
Analysis and quality assurance/genetic source, area, technology - development of "expected values" model.
The first stage of the program on the production of drug plants foresees monitoring of natural populations, their characterization and in situ conservation of evaluated autochthonous plant material.
MAP in situ conservation
In obtaining distribution data on wild accessions of MAP in Slovenia, Herbarium Ljubljana, literature data and floristic/vegetation inventory are used. The approach of phytogeographic regions has been proposed. In situ conservation of MAP is a part of genebank (ex situ) conservation strategy. Conservation of natural heredity for future generations and landscape attractiveness is the first scope of the work in conservation strategy in the field of MAP, and is planned to be achieved by:
Inventarization of natural resources and estimation of their endangerness in nature
Their active conservation (conservation in situ).
Reasonable and sustainable use of natural resources (limited on exploitation of germplasm for ex situ genebank
Prevention of massive exploration of natural resources through successive introduction of cultivation of known genotypes in suitable environment.
Selection and prebreeding of plant material in the direction of uniformity of chemical characteristics of plant raw material, requested by food and pharmaceutical industries are to be supported by the research work, based on characterization of germplasm and ecosystems, on inventarization of native populations still present in nature and maintenance of their global genetic diversity, evaluation, collecting of interesting "breeding" populations and utilization/introduction of germplasm in selection work/cultivar development. For efficient analysis and screening of interrelations between relevant parameters, observed on different research levels of each of the above-mentioned sectors, classic unidimensional databases were not sufficient. So, in order to manage this extensive data/information network, our working group has developed a relational database, which we called MEDPLANT (Baričevič et al. 1994).
MEDPLANT database has been established for floristic, faunistic, taxonomic and analytic data input. It's a managing tool for monitoring and evaluation of medicinal and aromatic plants (MAP) from the Central European, Southeastern and Mediterranean regions.
The main objectives of MEDPLANT are:
Screening of high potential genotypes for future selection/breeding work. This research -developmental level is based on comparison between chemical characteristics of selected ecotypes and between those chemical characteristics, that are essential for the market (i.e. quality requirements/standards).
Preservation of interesting populations of medicinal and aromatic plants (MAP) for future generations, based on monitoring of all natural MAP populations, and especially those at risk, and on reintroduction of clone material, if necessary.
Unification of documentation in research program/activities in European countries in the field of MAP.
Development of relevant descriptors, based on morphological and chemo-taxonomic characteristics of ecotypes and on the knowledge about their variability.
MEDPLANT system works on a relational database principles: collected data are arranged, according to their characteristics, into appropriate subdatabases (systematics, climatic region, geography, habitats, pedology, phytocoenoses, chemical analyses, varieties/cultivars, standards, market units...). Pointers, which connect specific data in these subdatabases enable establishing of mutual relationships between required data in the network.

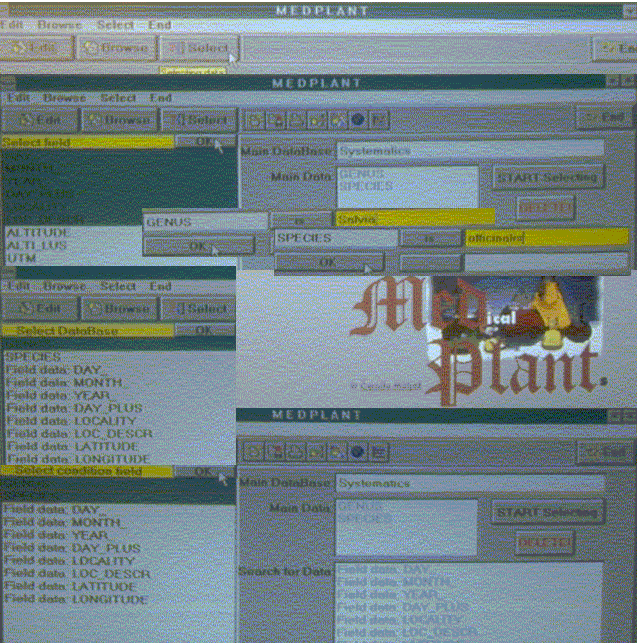
Insight in MEDPLANT relational database MEDPLANT system utilization enables input (two-step procedure: selection of key database for data input and selection of pertaining relational subdatabase for input of additional data) or review of data (three-step procedure: selection of key database for particular/required data, selection of pertaining subdatabase, search and additional selection of data according to the query) (Figure 1).
Considering the principle of sustainable use, natural populations of MAP (Crataegus monogyna Jacq., Plantago lanceolata L. Plantago major L., Pimipinella major (L.) Huds., Gentiana lutea L., Arnica montana L., Achillea millefolium L, Betonica officinalis L., Hypericum perforatum L., Hypericum montanum L., Rhamnus catharticus L., Origanum vulgare L., Ononis spinosa L., Epilobium parviflorum Schreber, Sedum maximum (L.) Krock, Satureja montana L., Anthyllis vilneraria L., Agrimonia eupatoria L., Lamium album L., Solidago virgaurea L., Centaurium erythraea Rafn, Urtica dioica L., Anthyllis vulneraria L., Malva sylvestris L., Eupatorium cannabinum L., Thymus serpyllum L, Potentilla erecta (L.) Räuschel, Euphrasia rostkoviana Hayne subsp. rostkoviana, Tussilago farfara L., Pimpinella saxifraga L., Veronica chamaedrys L., ....) are successively introduced in the National Collection of MAP, where further activities (multiplication of plant materials, morphological and/or chemical characterization, selection and other pre-breeding studies) needed for future cultivation purposes are foreseen.
MAP ex situ conservation
In 1996, the Slovenian Fund for Nature conservation ratified the Resolution on conservation of biological diversity and permanent landscape development, including MAP. In order to ensure gene-pools for future investigations, the genebank (a National Genebank Collection of medicinal and aromatic plants), that contains 650 autochthonous or foreign/introduced medicinal and aromatic plant accessions, has been set up in 1994, officially recognized in 1995 and is annually supported by Slovenian government. Ex situ gene bank aims at:
Maintenance of germplasm. The MAP accessions are maintained in the form of seeds (at +4°C or at -20°C) as plantations ex situ and as in vitro culture. Different propagation techniques are considered with the objectives of maintaining genetic source and of multiplication of plants for future field production (Cynara scolymus, Salvia officianlis, Origanum vulgare, Mentha piperita, Melissa officinalis, Hyssopus officinalis, Thymus vulgaris, Satureja montana, Hypericum perforatum, Gentiana lutea.....). Because of high morphologic and chemical variability in medicinal and aromatic plants in vitro culture technique has been applied for practical purposes, so the screening of optimal (considering velocity, morphological uniformity and low cost input) in vitro conditions is considered. The genebank accessions are successively introduced in the micropropagation procedure, where sterilization of plant material, culture media and rooting capacity are observed. After 6 years of experience, the micropropagation of medicinal and aromatic plants has been recognized as an essential tool in obtaining homogenous descendants in foreign pollinator species, in low-rate and long-period germinating species as well as in virus- infected plant material.
Evaluation of morphologic and chemotaxonomic characteristics of MAP (Origanum vulgare, Origanum hirtum).
Evaluation of medicinal and aromatic plant ecotypes for quantitative and qualitative differences in secondary metabolites with regard to growth and development (314 genebank accessions).
Evaluation of susceptibility of germplasm descendants to environmental stress (drought, low temperature, depleted soils,...) in pot trials under the conditions of controlled environment (Atropa belladonna L., Origanum vulgare L. ssp. hirtum, Satureja montana L., Thymus vulgaris L.,Trigonella foenum-graecum,...).
Small and medium - sized enterprises, galenical labs, and industry. Extraction of plant material and product finalization is foreseen.
Consideration of GMP, GLP, Rules governing medicinal products (EEC- 1989), WHO guidance on
MAP quality control (1998), validation of procedures.
Quality analysis and declaration of quality of raw materials and herbal products.
Documentation, approval/registration of products.
Aimed at protection of consumers/safety, efficacy, chemical quality.
Keeping the records/documentation about:
Cultivation areas /districts
Processing units/their capacities
Quality of drug plants on the market: domestic source, import.
Recommendations to farmers in regulation of quality.
Preparation of legislative regulations and trade control.
Supervision of implementation of NP/development of the MAP field (MEDPLANT).
Aljančič M, Gregorič J., Praprotnik N., Hlad B., Peterlin S., Skoberne P., Vidic J. (1995). Nature Conservation in Slovenia, Museum of Natural Science Ljubljana, 87 p.
Baričevič D., Černila M., Gomboc S., Habeler H. (1994). MEDPLANT - monitoring, characterization and evaluation software for medicinal and aromatic plants. SAA, Patent Nr. R 186/94, Ljubljana, Slovenija, 12 p.
Baričevič, D., Dedek, J., Bartol, T., Zupančič, A. (1996). Market possibilities of medicinal and aromatic plants in Slovenia. Proceedings of the International symposium- Novi izzivi c poljedelstvu (New challenges in Agriculture) '96, Biotechnical Faculty, p. 95-99.
Baričevič, D., Spanring, J., Činč, M, Umek, A., Stupica, T., Kus, M., Šuštar, F. (1994). National Program for cultivation, processing and quality control of drug plants and herbal remedies. Directives. University of Ljubljana, Biotechnical Faculty Ljubljana, 11 p.
Baričevič D. and Rode, J. (1996). National Program for cultivation, processing and quality control of drug plants and herbal remedies. Proposal. University of Ljubljana, Biotechnical Faculty and IHP Žalec, 12 p.
Wraber T., Skoberne P. (1989). The Red Data List of Threatened Vascular Plants in Slovenia.- Nature Conservation, 14-15, Institute for the Conservation of Natural and Cultural Heritage of Slovenia, Ljubljana.