|
|
 |
Two new sesquiterpene lactones with guaianolide skeleton (compounds 1 and 2) were isolated from the aerial parts of the flowering plant Anthemis carpatica Willd. (Asteraceae). The structure determination was based on the 1H NMR, IR, MS and 2D NMR methods - DQF COSY, TOCSY, PS NOESY, HSQC and HMBC.
Recently, in course of our chemotaxonomic studies of Yugoslavian plants belonging to Asteraceae, we have isolated from the aerial parts of Anthemis carpatica Willd. thirteen new highly oxygenated guaiadienolides, seven of them with a hydroperoxy function (1,2). Common features of these lactones were 11(13)-double bond, 10a-OH and 9a-OH (or OAcetyl) functionalities. Most of them, with exception of two, also contained 8a-OH (or Acyl functionality, Acyl = Acetyl or i-Butyryl). The remaining double bond was positioned in the five-membered ring in the following ways: (i) D2 (six compounds), (ii) D3 (four compounds) and (iii) D4 (three compounds). Before this, the only member of the genus containing hydroperoxy sesquiterepene lactones, together with hemiterpene and monoterpene hydroperoxy compounds was A. nobilis L. (syn. Chamaemelum nobile All.), the species, also known as Roman chamomile (3).
Due to a possible pharmacological importance of such highly oxygenated guaianolides, we are repeating the investigation of the aerial parts of A. carpatica (collected at the same locality as before, i.e. the north part of Šara mountain, Kosovo, July 1996), focusing on fractions containing lactones not isolated before.
General
CC: silica gel 60 (Merck), 0.063 - 0.200 mm; Kieselgel 60 GF254, layer thickness 0.25 and 1 mm; IR: transparent dry films; 1H NMR: CDCl3, CDCl3/CD3OD, at 200 and 300 MHz relative to TMS (at d = 0.00); 2D NMR spectra measured as usual (4); DCIMS double focusing (BE geometry).
Plant material
The plant material was collected during the flowering season (July 1996) at the north part of Šara Mountain (location Lavlja Vrata, altitude of ca. 1900 m). Voucher specimen (No 210796AC) was deposited in the herbarium of The Institute for Medicinal Plant Research "Dr. Josif Pančić", Belgrade.
Extraction and isolation
Crude extract (68.8 g) of air dried aerial parts (2115 g) was obtained by extraction with Et2O-petrol-MeOH (1:1:1) at room temperature, followed by treatment with MeOH to remove long-chain hydrocarbons, using usual procedure (5). The extract was subjected to CC silica gel, starting elution with petrol and gradually increasing polarity by addition of Et2O. Fraction eluted with petrol - Et2O (2:3) yielded by silica gel CC (toulene-Et2O-MeOH, 7:2:1) fractions I and II. Fraction I, after preparative TLC (toulene-Et2O-MeOH, 7:2:1, two developments), followed by repeated preparative TLC (CCl4-Et2O-MeOH, 5:4:1, four developments) afforded lactone 1 (3.3 mg). Lactone 2 (1.1 mg) was isolated by repeated CC (CHCl3-MeOH, 10:1), followed by preparative TLC (EtOAc-petrol-MeOH, 7:2.5:0.5, four developments).
Using the same extraction procedure as before (5), in combination with
silica gel CC and prep TLC, we have isolated two new guaianolides (1
and
2,
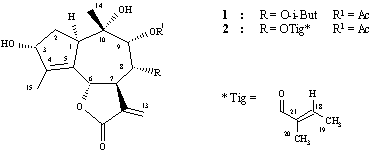
Figure 1) and identified by spectroscopic methods.
|
|
 |
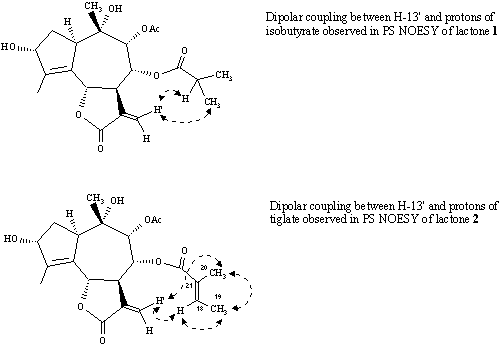
The correlation in NOESY between H-13' and the protons of these ester side chains, such as CH(CH3)2 (d 2.57, 1.20 and 1.19) from isobutyrate and H-18 from tiglate clearly indicate that in these cases the larger OAcyl group was in 8a-position, whereas the OAc group was in 9a-position. The NOEs, such as, H-14/H-6, H-14/H-8 and H-14/H-9 were also in agreement with the stereochemical proposal concerning these lactones (Figures 2 and 3).
The 1H NMR spectra of lactones 1 and 2 closely
resembling each other, were typical for the 8a,9a-diacyloxy-D4-guaianolides,
whose structure determination involving the application of various 2D NMR
techniques on 8a,9a-diacetoxy
analogues was reported previously (1). The common feature of lactones 1
and 2 was the acetoxy group (d 2.20)
whereas the nature of the remaining (larger) ester residue was evident
from the characteristic 1H NMR signals (Table 1).
|
|
 |
|
|
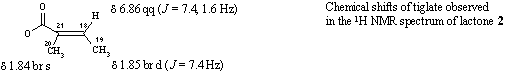 |
Spectral data of compound 1
8a-Isobutyryloxyanthemolide C (C21H28O8): colourless gum; [a]D25 + 510 (MeOH; c 0.14); IR vmax cm-1 : 3396 (OH), 1741 (C=O, a,b-unsat. g-lactone, esters), 1655 (C=C); 1H NMR (See Table 1); DCIMS (isobutane, 150 eV) m/z (rel. int.): 409 [M+H]+ (12), 391 [M+H-18]+ (100), 373 [M+H-2x18]+ (42), 349 [M+H-60]+ (10), 321 [M+H-88]+ (53), 303 [M+H-18-88]+ (53), 285 [M+H-2x18-88]+ (10).
Spectral data of compound 2
8a-Tigloyloxyoxyanthemolide C (C22H28O8): colourless gum; [a]D25 + 570 (MeOH; c 0.11); IR vmax cm-1 : 3418 (OH), 1753 (C=O, a,b-unsat. g-lactone, OAc), 1725 (C=O, OTig), 1650 (C=C); 1H NMR (See Table 1); DCIMS (isobutane, 150 eV) m/z (rel. int.): 421 [M+H]+ (9), 403 [M+H-18]+ (100), 385 [M+H-2x18]+ (32), 361 [M+H-60]+ (5), 321 [M+H-100]+ (92), 303 [M+H-18-100]+ (53), 285 [M+H-2x18-100]+ (18).
The major structural feature of isolated sesquiterpene lactones, such as a,b-unsaturated-g-lactone moiety, has been shown to be associated with their pharmacological properties. In a study of the structure-activity relationship among these bitter substances, it was noted that the presence of 11(13)-exocyclic double bond conjugated to the g-lactone was essential for cytotoxicity. It could be worth efforts to continue with the further examination regarding the biological activities of these lactones.
|
|
|
|
|
|
ca. 3.74 m | ca. 3.74 m |
|
|
1.91 ddd (14.8, 6.9, 2.4) | 1.87 ddd (14.8, 6.9, 2.4) |
|
|
ca. 2.3 m | ca. 2.3 m |
|
|
4.62 br d (7.6) | 4.57 br d (7.6) |
|
|
4.81 dq (10.8, 1.6) | 4.86 dq (10.8, 1.6) |
|
|
3.69b dddd (10.8, 10.0, 3.6, 2.6) | 3.70b dddd (10.8, 10.0, 3.6, 2.6) |
|
|
5.24 br d (10.0) | 5.32 br d (10.0) |
|
|
5.29 br s | 5.28 br s |
|
|
5.90 br d (2.6) | 5.80 br d (2.6) |
|
|
6.34 d (3.6) | 6.28 d (3.6) |
|
|
1.10 s | 1.08 s |
|
|
1.99 t (1.6) | 1.98 t (1.6) |
|
|
2.20 s | 2.19 s |
|
|
--- | 2.57 sep (7.2) 1H
1.20 d (7.2) 3H 1.19 d (7.2) 3H |
|
|
H-18, 6.86 qq (7.4, 1.6) 1H
H-20, 1.84 br s H-19, 1.85 br d (7.4) 3H |
--- |
The authors are grateful to the Ministry for Science and Technology, Republic of Serbia for financial support.
Bulatović V., Vajs V., Macura S., Juranić N., Milosavljević S. (1997): Highly Oxygenated Guaianolides from Anthemis carpatica. J. Nat. Prod 60, 1222-1229.
Bulatović V., Vajs V., Milosavljević S., Menković N., Šavikin-Fodulović K. (1997) New Sesquiterpene Lactone from Anthemis carpatica, C26, 45th Annual Congress on the Society for Medicinal Plant Research, Regensburg.
Rücker G., Mayer R., Lee K.R. (1989): Peroxides as Plant Constituents, VI: Hydro-peroxides from the blossoms of Roman Camomille (Anthemisnobilis L., Asteraceae). Arch. Pharm. (Weinheim) 322, 821-826.
Milosavljević S., Vajs V., Bulatović V., Đoković D., Aljančić I., Macura S., Juranić N. (1998): Application of two-dimensional nuclear magnetic resonance methods for structure elucidation of sesquiterpene lactones (guaianolides) from Anthemis carpatica and diterpene from Achillea clypeolata. Recent Research Developments in Phytochemistry 2, 383-395.
Bohlmann, F., Zdero, C., King, H. R., Robinson, E. H. (1984): Pseudoguaianolides and other sesquiterpene lactones from Gaillardia species. Phytochemistry 23, 1979-1988.