|
|
 |
Withania somnifera in vitro cultures were initiated using as explant resources the biological material from the Iasi Botanical Gardens. Shoot tips, nodes and leaves harvested from in vitro obtained neo-plantlets were cultivated on MS medium supplemented with hormonal formulae which induced callus formation. The TLC of the dichlormethane, methanolic and water extracts showed that the obtained callus had the capacity to synthesise triterpenes, phytosterols, flavones, polyphenolcarboxilic acids, alkaloids and polysaccharides. The biosynthetic potential of the callus depended on the explant we used, age of callus, hormonal composition of the nutrition medium.
A plant with a very extended areal - from the Atlantic Ocean to Southeast Asia (1,3,12) - Withania somnifera Dun. is known in traditional medicine for following proprieties of the drug: tonic, hypnotic, analgesic, antipyretic, antibiotic, hypertensive, anti-inflamatory, sedative, aphrodisiac, anti-tumoral etc. (2,3,11,12,14-16), (especially the roots). Some of these proprieties are used in modern medicine. The mentioned effects are due to the alkaloids and withanolids. The drug of Withania somnifera also contains tanins, polyphenols, glycosides, fatty acids, polysaccharides, an alkaloid similar to reserpine, essential oils, etc. (3,12,16). As withaferine A, withanolids D and E proved to have an antitumoral activity (5,13), it was synthesised some of these and tested their efficiency.
Learning of the above-mentioned qualities we investigated the plant in the "Stejarul" Research Centre, Piatra Neamt. Up to now we outlined the morphogenetic reaction of Withania somnifera explants on different nutrition media, we evaluated the quantity of the accumulated biomass, in a certain time interval, by organogene and callus cultures, we comparatively studied - in field cultures - some morpho-physiological at the end of the ontogenesis of the plants, obtained generatively and from in vitro cultures. Along these, we elaborated a technology of species micropropagation, obtained information on the behaviour of the phytochemical and morpho-physiological indices of plants originating from seeds irradiated with different gamma rays doses, etc, (6-10). The paper presents information referring to the biosynthetic capacity of some active principles in Withania somnifera callus.
To know the biosynthetic potential of Withania somnifera callus we cultivated explants - shoot tips, nodes and leaves from in vitro regenerated neo-plantlets - on MS (1962) supplemented with different concentrations of cytokinine and auxine. The morpho-physiological peculiarities of the callus obtained on the 8 experimental variants are presented in Table 1. The phytochemical analyses aimed to show the influence of the nutritive growth regulator composition, origin of the explant and the callus age on some classes of active principles. To perform the qualitative phytochemical analyses, callus samples were dried at 40°C, grinned and than extracted with dichormethane, methanol and water. The extracts were reduced to a volume of 1 ml out of which we used 50 ml for chromatography. We used the TLC method with Fertigplatten Kieselgel G60 (10x20 cm) for triterpenes and polyphenolic derivatives and F254 (10x20 cm) for polysaccharides. The results are presented in Tables 1-2, and Figures 1-3.
Our previous investigations showed that the initiation of in vitro cultures of Withania somnifera Dun. may successfully be performed using as explants aseptically germinated plantlets or apical and axilary shoot tips, inoculated on MS supplemented with small quantities of cytokinins. Callus induction may also be performed on greater quantities of cytokinins, but is optimal on media including auxins or the combination of the two (in favour of the second) (7). In this experiment, the tested hormonal formula assured only callus induction and in one case (Var. 3, Table 1) a slight differentiation process (of the roots). The best capacity of cell proliferation was present in the callus obtained from shoot tips inoculated on MS supplemented with BAP and NAA. The callus obtained from node has a superior content of dry substance (related to the fresh substance) compared to the callus obtained from leaves and shoot tips. It also seams that the ageing callus looses the capacity to retain water, having - as such - a higher content of dry weight.
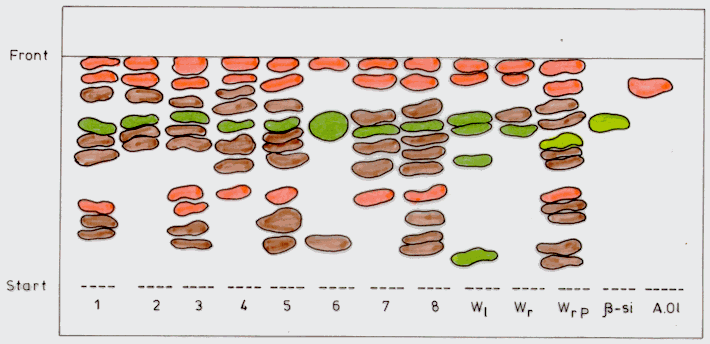
The chromatographic analysis of the dichlormethane extract triterpenes (Figure 1) showed a large spectrum in the callus samples, richer than in the leaves and roots of the plants grown in the field of the "Stejarul" Research Centre, Piatra Neamt. It is to be noted that variants 3, 5 and especially 8 have a triterpene spectrum (10-11 compounds) similar to that in the roots of the Pakistanese plants (12 compounds). Thus, there is the possibility to obtain, in Withania somnifera callus cultures, triterpene compounds specific to the roots of the plants in the natural area of the species. We also found that the biosynthesis of these compounds greatly depends not so much on the origin of the explant, but on the hormonal composition of the medium. The presence of BAP assured the synthesis of a larger spectrum of triterpenes in the generated callus. A proof is sample 6, the callus of which was obtained on a medium without BAP, which has only 3 triterpenes. The nature of the explant also seems to have an influence, in the respect that in the calli obtained from shoot nodes we identified a smaller range of triterpenes that in those obtained from leaves and shoot tips. All the callus samples had oleanolic acid and b-sitosterol (Figure 1). An exception is sample 6, which had no oleanolic acid.
|
|
|
|
capacity |
|
|
|
|
shoot nodes | cream-coloured-brown compact | relatively slow
no organogenesis |
|
|
|
|
shoot nodes | compact, globulous-discoidal | reduced growth |
|
|
|
|
shoot tips | compact, whitish-cream-coloured white light, brownish areas | active proliferation |
|
|
|
|
shoot tips | compact, light-cream-coloured, brownish at the edges, rarely rhysogenesis | active proliferation |
|
|
|
|
shoot tips | compact, irregular surface | slow growth |
|
|
|
|
shoot nodes | brown-cream-coloured, less compact | slow growth |
|
|
|
|
shoot nodes | compact, light-brown-cream-coloured | horizontally growth |
|
|
|
|
leaves | relatively compact, dark cream-coloured | slow proliferation |
|
|
BAP=2 mg/l BAP; BN=1 mg/l BAP and 1 mg/l NAA; BDN=0.5 mg/l BAP, 0.5
mg/l 2.4-D and 0.5 mg/l NAA; BD=1-2 mg/l BAP and 0.5 mg/l 2.4-D; DN=0.5
mg/l 2.4-D and 0.5 mg/l NAA.
|
|
 |
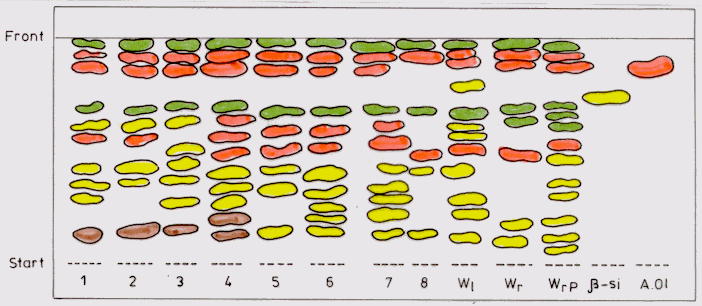
The spectrum of triterpenes identified by TLC in methanolic extract
is richer and more uniform in the analyses callus samples compared to the
dichormethanic extract. An exception is sample 8 (leaf callus obtained
on MS with BAP and 2,4-D) which has only 6 triterpene compounds (Figure
2). A great number of triterpenes are contained by samples 4, 6 and 7 (11-12
compounds), close to the one identified in the leaves of the field cultivated
plants and in the Pakistanese roots. This time, b
-sitosterol is present only in the leaves, while oleanolic acid is in all
the callus samples, except sample 8 (Figure 2).
|
|
 |
|
|
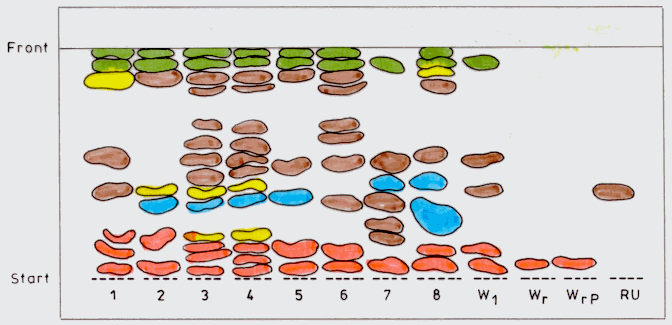 |
Important differences between the analysed samples appear in the respect of polyphenolic derivatives identified in methanolic extract (Figure 3), the number ranging in large limits (from 7 to 14, depending on the nature of the callus). The greatest number of polyphenolic derivatives are in samples 3 and 4 (14 compounds), which represent shoot tip calli of 6 weeks of age on MS supplemented with BAP and NAA. Except these 2 samples, having an identical spectrum of polyphenolic derivatives, each of the other 6 analysed callus samples has a characteristic spectrum. The majority of the identified compounds belong to the flavonosides. Among these, rutosid is present in sample 1, the other samples having flavonic derived but not the rutosid. Samples 2, 3, 4, 5 and 7 have one polyphenolcarboxilic acid, sample 8 has two such compounds, while samples 1 and 6 have no such acids (Figure 3).
Analysing the alkaloids of Pakistanese roots, El Sakka (1991) found one alkaloid like reserpine (3). Our investigations showed that in the dichlormethane extract, except callus samples 4 and 8, all the others have this alkaloid. In methanolic extract only sample 8 did not have this alkaloid. In the other samples we had 1-3 spots characteristic to choline and its derivatives.
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Glucose |
|
|
|
|
|
|
|
|
|
|
|
| Galactose |
|
|
|
|
|
|
|
|
|
|
|
| Arabinose |
|
|
|
|
|
|
|
|
|
|
|
| Rhamnose |
|
|
|
|
|
|
|
|
|
|
|
| Xylose |
|
|
|
|
|
|
|
|
|
|
|
| Glucuronic acid |
|
|
|
|
|
|
|
|
|
|
|
| Galacturonic acid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Rf = 0.85 |
|
|
|
|
|
|
|
|
|
|
|
| Rf = 0.78 |
|
|
|
|
|
|
|
|
|
|
|
| Rf = 0.45 |
|
|
|
|
|
|
|
|
|
|
|
| Rf = 0.40 |
|
|
|
|
|
|
|
|
|
|
|
The polysaccharide fraction obtained in water extract was made up of glucose, galactose, arabinose, ramnose, xylose, glucuronic acid, galacturonic acid and other 4 unidentified monomers (because of lack of standards), for which we determined the Rf. From the data of Table 2 we find that the polysaccharide fraction in calli is more complex than that extracted from the leaves and roots of the field grown plants. The callus obtained on MS supplemented with 2,4-D and NAA (sample 6) registered the most complex composition of this fraction. There are slight differences from the point of view of the composition in monomers of the polysaccharide fraction (see Table 2).
Callus of Withania somnifera Dun. obtained on different hormonal formulae of the MS had different morpho-physiological peculiarities and chemical composition.
The qualitative phytochemical analyses showed the capacity of the obtained callus to synthesise some groups of active principles such as: triterpenes, polyphenolic derivatives (especially flavones), and alkaloids. The spectrum of these substances depended on the nature of the explant, the hormonal composition of the nutrition medium, callus type, etc. being frequently larger in the callus samples compared to the leave and root samples of the field grown plants.
The polysaccharide fraction of the water extract of the callus was made up of: glucose, galactose, arabinose, ramnose, xylose, glucuronic acid, galacturonic acid and other 4 unidentified monomers.
The future quantitative phytochemical analyses will show us the possibilities to use the callus in order to obtain some active principles specific to the plant.
Ascher K.R.S., Eliahu M., Glotter E., Kirson I., Abraham A. (1984): Phytoparasitica, 12, 3-4, 147-155.
Bhatrager S.S. (1976): The wealth of India, X, 58, 581-585.
El Sakka M.A. (1991): Contributii la valorificarea speciei Withania somnifera Dun., Teza de doctorat, U.M.F. Iasi, 199 p.
El Sakka M.A. (1995): Methodology of medicinal plant research, Edit. Tehnica, Chisinau, 193 p.
Gamoh K., Hirayama M., Ikekawa N. (1984): J. Chem. Soc. Perkin Trans., 1, 449-454.
Ghiorghita G., Gille E., El Sakka M.A. (1992): Proceed. 2-nd Nat. Congress of Biology, Iasi, Sept. 16-20, 137-138.
Ghiorghita G., El Sakka M.A., Stanescu U., Gille E. (1994): Abstr. VII-th Int. Congress of IAPTC, Firenze, June 12-17, S.18-74.
Ghiorghita G., Gille E., Prisecaru M., Nicuta D., Pislariu I.C.: Aspects of the in vitro behavior of Withania somnifera Dun. I. Callus induction and organogenesis, Studii si cercet. (Univ. Bacau). In print.
Ghiorghita G., Gille E., Pislariu I.C., Nicuta D.: Aspects of the in vitro behaviour of Withania somnifera Dun. II. Biomass accumulation in in vitro and ex vitro cultures, Rev. Roum. Biol., Ser. Biol. Végét. In print.
Ghiorghita G., Gille E., Ichim M.C., Nicuta D. (1999): Romanian J. Biol. Sci., 3, 9-10.
Jaffer J.M., Jawad M.A.L., Saber S.H., Al-Nail A. (1988) - Fitoterapia, 59, 6, 497-500.
Kirtikar R.K. and Basu B.D. (1981): Indian Medicinal Plants, Dehradun, Delhi, III, 1774-1779.
Moriguchi I. and Komatsu K. (1981):Eur. J. Med.Chem., Chimica Therapeutica, 1, 19-23.
Nedkarni K.M. and Nedkarni A.K. (1976): The Indian Materia Medica, 1, 1292-1294.
Yidya Prabhu M. and Rao A. (1990): Fitoterapia, 59, 3, 237-240.
Watt J.M. and Brandwijk M.G. (1962): Medicinal poisonous plants of Southern and Eastern Africa, 2nd Edit., E. and S. Livingstone LTD London, 1010-1012.